Precision manufacturing demands perfection, and choosing between cleanroom and clean-process micro-manufacturing can determine your product’s success, quality standards, and operational costs.
🔬 The Evolution of Contamination-Free Manufacturing
Modern manufacturing has reached unprecedented levels of sophistication, particularly in industries where microscopic particles can spell disaster. From semiconductor fabrication to pharmaceutical production, the battle against contamination has driven innovation in two distinct approaches: traditional cleanroom environments and emerging clean-process manufacturing techniques.
Understanding the fundamental differences between these methodologies is crucial for manufacturers seeking to optimize their operations. Both approaches aim to eliminate contaminants, but they achieve this goal through remarkably different philosophies and practical implementations. The cleanroom approach creates an entirely controlled environment, while clean-process manufacturing focuses on isolating specific production steps from contamination sources.
The global cleanroom technology market continues to expand, driven by stringent regulatory requirements and increasing demand for high-precision products. Meanwhile, clean-process manufacturing represents an innovative alternative that challenges conventional wisdom about contamination control.
🏭 Cleanroom Manufacturing: The Gold Standard Explained
Cleanroom manufacturing operates on a comprehensive environmental control principle. These specialized facilities maintain strictly regulated conditions for temperature, humidity, air pressure, and most critically, particulate contamination levels. The International Organization for Standardization (ISO) classifies cleanrooms from ISO 1 (the cleanest) to ISO 9, with each class representing a tenfold increase in permitted particle concentration.
The architecture of cleanroom facilities incorporates advanced HEPA or ULPA filtration systems that continuously circulate and purify air. Positive air pressure prevents external contaminants from entering, while specialized airlocks and gowning procedures minimize particle introduction through human activity. Workers must don complete protective garments including coveralls, gloves, masks, and shoe covers before entering production areas.
Critical Components of Cleanroom Infrastructure
Several interconnected systems work together to maintain cleanroom integrity. Air handling units process thousands of cubic feet per minute, creating laminar flow patterns that sweep particles away from critical work surfaces. Environmental monitoring systems continuously track particle counts, temperature, humidity, and pressure differentials, triggering alarms when parameters drift outside acceptable ranges.
Surface materials within cleanrooms require careful selection. Non-porous, easy-to-clean materials like stainless steel and specialized polymers dominate cleanroom construction. Even seemingly minor details like rounded corners instead of sharp angles help prevent particle accumulation in hard-to-clean areas.
Industries Dependent on Cleanroom Technology
- Semiconductor and microelectronics fabrication requiring sub-micron precision
- Pharmaceutical manufacturing of sterile injectable products and biologics
- Medical device production for implantable and invasive equipment
- Aerospace component manufacturing for satellite and spacecraft systems
- Optics and photonics for precision lenses and laser components
- Biotechnology research facilities handling sensitive cell cultures
⚙️ Clean-Process Manufacturing: The Targeted Alternative
Clean-process manufacturing represents a paradigm shift from environment-wide contamination control to process-specific protection. Rather than creating an entirely controlled space, this approach identifies critical contamination points within the manufacturing workflow and implements targeted protection measures at those specific stages.
This methodology acknowledges that not every manufacturing step requires the same level of environmental control. By focusing resources on the most contamination-sensitive operations, clean-process manufacturing can achieve comparable results to cleanroom production while potentially reducing infrastructure costs and operational complexity.
Isolation technologies form the backbone of clean-process manufacturing. Mini-environments, enclosed workstations, and process-specific barriers create localized zones of extreme cleanliness exactly where needed. Advanced robotics and automation further minimize human intervention during critical manufacturing stages, reducing contamination risk without requiring extensive facility-wide controls.
Technological Enablers of Clean-Process Methods
Modern clean-process manufacturing leverages several sophisticated technologies. Laminar flow hoods create particle-free zones around specific workpieces without controlling the entire room environment. Glove boxes and isolators allow manipulation of sensitive materials while maintaining complete separation from ambient conditions. Automated material handling systems transport components between process steps without human contact or exposure to uncontrolled environments.
Real-time monitoring systems track contamination at critical control points rather than throughout an entire facility. This targeted approach generates more relevant data about actual process cleanliness while reducing the volume of environmental monitoring required. When contamination events occur, pinpointing the source becomes more straightforward because monitoring focuses on specific high-risk operations.
💰 Cost Analysis: Investment and Operational Expenses
Financial considerations often determine which manufacturing approach organizations adopt. Cleanroom facilities demand substantial capital investment, with construction costs ranging from hundreds to thousands of dollars per square foot depending on classification requirements. ISO 5 cleanrooms (Class 100), commonly used in semiconductor manufacturing, represent some of the most expensive facilities to build and operate.
Operational expenses for cleanroom facilities extend well beyond initial construction. Energy consumption for continuous air filtration and environmental control can represent 30-50% of operating costs. Regular maintenance of HVAC systems, filter replacements, and facility certification add ongoing expenses. Personnel costs increase due to gowning requirements, specialized training, and reduced productivity from access procedures.
Comparative Cost Structure
| Cost Category | Cleanroom Manufacturing | Clean-Process Manufacturing |
|---|---|---|
| Initial Capital Investment | Very High ($1,000-$3,000/sq ft) | Moderate ($200-$800/sq ft) |
| Energy Consumption | Extremely High (continuous) | Moderate (targeted systems) |
| Maintenance Requirements | Extensive (facility-wide) | Focused (specific equipment) |
| Personnel Training | Comprehensive protocols | Process-specific procedures |
| Scalability Costs | Exponential expansion expenses | Linear scaling potential |
Clean-process manufacturing typically requires lower initial investment because it doesn’t demand complete facility transformation. Capital expenditure focuses on specific equipment like isolation chambers, localized filtration systems, and automated handling equipment. While individual pieces of advanced isolation technology can be expensive, the total investment usually remains substantially below full cleanroom construction costs.
🎯 Performance Metrics: Quality and Precision Outcomes
Both methodologies can achieve exceptional contamination control when properly implemented, but their performance characteristics differ in important ways. Cleanroom manufacturing provides consistent, predictable environmental conditions throughout the production space. This uniformity simplifies process validation and quality assurance because environmental variables remain tightly controlled across all operations.
Defect rates in properly maintained cleanroom facilities typically reach extremely low levels. Semiconductor fabs operating at ISO 4 or better routinely achieve yields exceeding 95% for complex integrated circuits. Pharmaceutical cleanrooms consistently meet regulatory standards for sterile product manufacturing with contamination rates measured in parts per million or billion.
Clean-process manufacturing demonstrates comparable performance for many applications, particularly when contamination risk concentrates at specific production stages. Advanced isolation technologies can create localized environments cleaner than the surrounding space, sometimes achieving ISO 3 or better conditions within mini-environments located in conventional manufacturing areas.
Quality Assurance Considerations
Validation and regulatory compliance present different challenges for each approach. Cleanroom facilities benefit from established standards and well-understood qualification procedures. Environmental monitoring follows standardized protocols, and regulatory agencies have extensive experience evaluating cleanroom operations. This regulatory familiarity can streamline approval processes for industries like pharmaceuticals and medical devices.
Clean-process manufacturing requires more sophisticated validation strategies because contamination control occurs at discrete points rather than continuously throughout a space. Process validation must demonstrate that targeted protection measures adequately control contamination risks. While this approach can be equally effective, it may require more extensive documentation and risk assessment to satisfy regulatory requirements.
🔄 Flexibility and Scalability: Adapting to Manufacturing Demands
Manufacturing agility has become increasingly important as product lifecycles shorten and customization demands grow. Cleanroom facilities offer limited flexibility once constructed. Modifying cleanroom layouts, expanding production capacity, or repurposing spaces for different products requires significant time and investment. The integrated nature of cleanroom systems means changes to one area often affect adjacent spaces.
Production volume scalability in cleanroom environments typically requires substantial planning and capital investment. Expanding capacity might mean constructing entirely new cleanroom suites, a process taking months or years and consuming millions in capital expenditure. This long lead time can disadvantage organizations in rapidly evolving markets.
Clean-process manufacturing generally offers superior flexibility. Isolation equipment and mini-environments can be relocated, reconfigured, or repurposed with relative ease compared to fixed cleanroom infrastructure. Adding production capacity might involve installing additional isolation chambers or process-specific protection equipment rather than expanding entire controlled environments.
Product Changeover Efficiency
Transitioning between different products or production runs demonstrates another flexibility advantage for clean-process approaches. Cleanroom changeovers require thorough cleaning and re-certification of entire production areas, potentially taking days or weeks. Clean-process systems isolate individual operations, allowing faster, more targeted cleaning and validation of specific equipment rather than entire rooms.
🌍 Environmental Impact and Sustainability Considerations
Sustainability concerns increasingly influence manufacturing decisions. Cleanroom facilities consume enormous amounts of energy, primarily for continuous air circulation, filtration, and environmental control. A typical pharmaceutical cleanroom uses 10-20 times more energy per square foot than conventional office space. This energy intensity translates directly into substantial carbon footprints and operational costs.
Water consumption for humidity control, equipment cooling, and cleaning procedures adds another environmental consideration. Cleanroom operations also generate significant waste streams including spent filters, single-use protective garments, and cleaning materials. While necessary for contamination control, these disposables contribute to environmental impact.
Clean-process manufacturing typically demonstrates improved environmental performance. Targeted contamination control reduces overall energy consumption because only specific process areas require intensive environmental management. Smaller controlled volumes mean less air to filter, fewer protective garments to dispose of, and reduced resource consumption across multiple categories.
🚀 Emerging Technologies Reshaping Contamination Control
Innovation continues transforming both manufacturing approaches. Advanced materials like self-cleaning surfaces and antimicrobial coatings reduce contamination accumulation in production environments. Nanotechnology-based filtration systems achieve higher efficiency with lower energy consumption compared to conventional HEPA filters.
Artificial intelligence and machine learning optimize contamination control strategies in real-time. Predictive analytics identify potential contamination events before they occur, allowing preemptive intervention. Smart sensors continuously monitor environmental conditions and process parameters, automatically adjusting protection systems to maintain optimal cleanliness.
Hybrid approaches combining cleanroom and clean-process philosophies represent an emerging trend. These systems maintain moderate facility-wide environmental control while implementing enhanced protection at critical process steps. This layered defense strategy balances the advantages of both approaches while mitigating their respective limitations.
📊 Making the Strategic Choice: Decision Framework
Selecting between cleanroom and clean-process manufacturing requires careful analysis of multiple factors. Product requirements provide the starting point—what contamination levels can your products tolerate? Some applications demand comprehensive environmental control, while others perform adequately with targeted protection.
Regulatory requirements significantly influence this decision. Highly regulated industries like pharmaceuticals often find cleanroom facilities essential for regulatory compliance, despite higher costs. Less regulated sectors may have more flexibility to implement innovative clean-process approaches.
Production volume and product mix affect the equation. High-volume, single-product manufacturing may justify cleanroom investment, while diverse, lower-volume production might benefit from clean-process flexibility. Future expansion plans matter too—organizations anticipating rapid growth should consider scalability advantages carefully.
Risk Tolerance and Quality Philosophy
Organizational risk tolerance plays a crucial role. Conservative organizations in established industries may prefer cleanroom manufacturing’s proven track record despite higher costs. More agile companies in emerging sectors might embrace clean-process innovation to gain competitive advantages through lower capital requirements and operational flexibility.
The decision ultimately depends on balancing quality requirements, regulatory constraints, financial resources, operational preferences, and strategic objectives. Neither approach universally “reigns supreme”—the optimal choice depends entirely on specific organizational circumstances and manufacturing requirements.
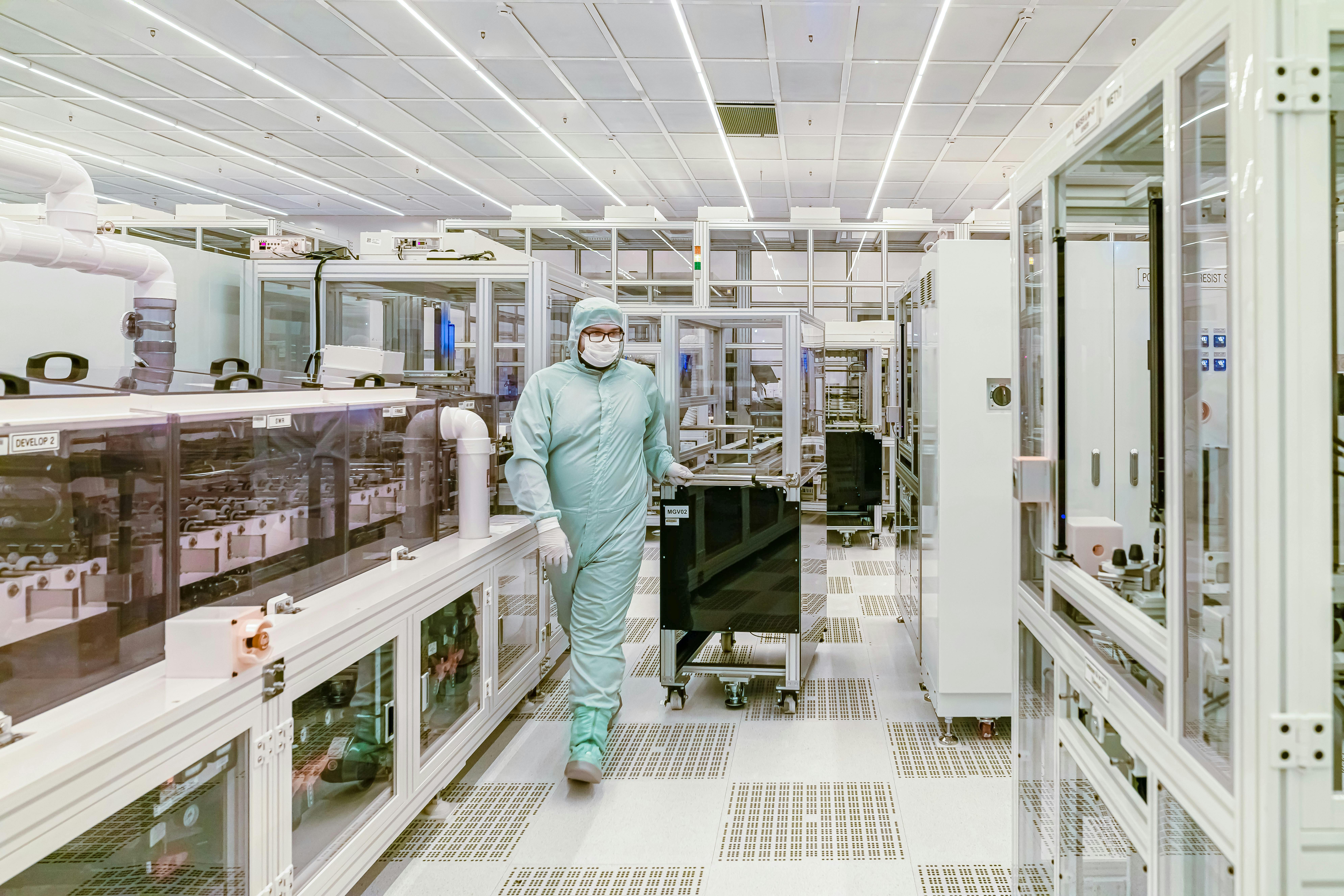
🎓 The Future Landscape of Precision Manufacturing
Both cleanroom and clean-process manufacturing will continue evolving and coexisting in the precision manufacturing ecosystem. Cleanroom technology will advance through improved energy efficiency, smarter environmental control systems, and integration with Industry 4.0 concepts. These facilities will likely become more sustainable and cost-effective while maintaining their exceptional contamination control capabilities.
Clean-process manufacturing will expand its applicability as isolation technologies improve and regulatory acceptance grows. As more organizations demonstrate successful implementation, validation protocols will become standardized, reducing adoption barriers. The approach particularly suits emerging manufacturing paradigms like distributed production, mass customization, and rapid prototyping.
The most successful manufacturers will likely adopt flexible contamination control strategies tailored to specific products and processes. Rather than viewing these approaches as competing alternatives, forward-thinking organizations will leverage elements of both methodologies to optimize quality, cost, flexibility, and sustainability simultaneously.
Understanding the strengths and limitations of cleanroom versus clean-process micro-manufacturing empowers informed decision-making that aligns contamination control strategies with broader business objectives. As manufacturing continues advancing toward greater precision, customization, and sustainability, the organizations that thoughtfully select and implement appropriate contamination control approaches will gain decisive competitive advantages in increasingly demanding markets. 🌟
Toni Santos is a manufacturing systems researcher and sustainable production specialist focusing on carbon-neutral materials, clean micro-manufacturing processes, digital precision machining, and sustainable batch systems. Through an interdisciplinary and efficiency-focused lens, Toni investigates how advanced manufacturing can integrate ecological responsibility, precision engineering, and resource optimization — across industries, scales, and production paradigms. His work is grounded in a fascination with manufacturing not only as production, but as carriers of environmental impact. From carbon-neutral material innovation to clean micro-manufacturing and digital precision systems, Toni uncovers the technical and operational tools through which industries can achieve their transition toward sustainable production practices. With a background in manufacturing engineering and sustainable production systems, Toni blends technical analysis with environmental research to reveal how materials can be sourced responsibly, machined precisely, and processed sustainably. As the creative mind behind fynvarox, Toni curates precision manufacturing insights, carbon-neutral material studies, and sustainable batch system strategies that advance the integration between industrial efficiency, digital accuracy, and ecological integrity. His work is a tribute to: The responsible sourcing of Carbon-Neutral Materials and Processes The precision methods of Clean Micro-Manufacturing Technologies The accuracy and control of Digital Precision Machining The resource-efficient design of Sustainable Batch Production Systems Whether you're a manufacturing engineer, sustainability researcher, or curious practitioner of responsible production, Toni invites you to explore the future of clean manufacturing — one material, one process, one system at a time.




